Battery

Various cells and batteries (top-left to bottom-right): two
AA, one
D, one handheld
ham radiobattery, two
9-volt (PP3), two
AAA, one
C, one
camcorder battery, one
cordless phone battery
Type Power source
Working principle Electrochemical reactions,
Electromotive force
First production 1800s
Electronic symbol
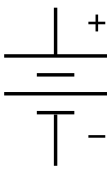
The
symbol for a battery in a
circuit diagram. It originated as a schematic drawing of the earliest type of battery, a
voltaic pile.
An electric
battery is a device consisting of one or more
electrochemical cells with external connections provided to power electrical devices such as
flashlights,
smartphones, and
electric cars.
[1] When a battery is supplying
electric power, its positive terminal is the
cathode and its negative terminal is the
anode.
[2] The terminal marked negative is the source of electrons that when connected to an external circuit will flow and deliver energy to an external device. When a battery is connected to an external circuit,
electrolytes are able to move as ions within, allowing the chemical reactions to be completed at the separate terminals and so deliver energy to the external circuit. It is the movement of those ions within the battery which allows current to flow out of the battery to perform work.
[3]Historically the term "battery" specifically referred to a device composed of multiple cells, however the usage has evolved additionally to include devices composed of a single cell.
[4]
Primary (single-use or "disposable") batteries are used once and discarded; the
electrode materials are irreversibly changed during discharge. Common examples are the
alkaline battery used for
flashlights and a multitude of portable electronic devices.
Secondary (rechargeable) batteries can be discharged and recharged multiple times using an applied electric current; the original composition of the electrodes can be restored by reverse current. Examples include the
lead-acid batteries used in vehicles and
lithium-ion batteries used for portable electronics such as
laptops and
smartphones.
Batteries come in many shapes and sizes, from miniature cells used to power
hearing aids and wristwatches to small, thin cells used in
smartphones, to large
lead acid batteries used in cars and trucks, and at the largest extreme, huge battery banks the size of rooms that provide standby or emergency power for
telephone exchanges and computer
data centers.
According to a 2005 estimate, the worldwide battery industry generates US$48
billion in sales each year,
[5] with 6% annual growth.
Batteries have much lower
specific energy (energy per unit mass) than common
fuels such as gasoline. In automobiles, this is somewhat offset by the higher efficiency of electric motors in producing mechanical work, compared to combustion engines.


